In an attempt to stop the spread of the coronavirus disease (COVID-19), countries have been implementing “mass testing” to their general population. But in the Philippines, the opposite is true.
The planned mass testing is “targeted” to a certain population, the Department of Health (DOH) said April 4.
Insufficient medical resources, lack of healthcare professionals and accredited testing centers, leave the Philippines lagging behind other countries in early virus detection.
According to a tracker by the University of the Philippines (UP), around 6,000 individuals were tested by the first week of April, with more than 3,000 COVID-19 positive patients.
With more than 100 million Filipinos, these figures barely scratch the surface.
Backlog on test kit production
After the local transmission of the virus was confirmed early March, the Research Institute for Tropical Medicine (RITM) said the country initially had 2,000 test kits. Weeks later, 168,000 test kit donations poured out from China, Singapore and South Korea.
These supplies combined, however, could not test even five percent or more than 5 million of the population, which the susceptible aged 65 and above comprise.
Recently, the Philippine Red Cross completed its first testing lab with the capacity to process 3,000 tests a day.
DOH reported April 6 that nearly 23,000 COVID-19 tests have already been conducted. However, this doesn’t mean one test is equivalent to one person, since those diagnosed with COVID-19 have to be tested at least three times. Aside from the confirmatory test, the patient must also be tested twice before being discharged from the hospital.
In other countries, the production of kits and mass testing came right before the crisis shoot up in early March. As soon as Chinese scientists published the virus’ genetic sequence in January, at least four South Korean firms began to develop test kits. South Korea tested 10,000 people daily on-site and via drive-thrus and had conducted over 190,000 tests by as early as March 9.
Germany had already produced 4 million test kits by the end of February after a local scientist, Olfert Landt, saw the similarity of the novel coronavirus to the 2003 Severe Acute Respiratory Syndrome.
Who qualifies for testing?
The National Task Force (NTF) against COVID-19 Chief Implementer Carlito Galvez said the government is already preparing to carry out “massive testing” but only for Persons Under Monitoring (PUMs), Persons Under Investigation (PUIs) and health workers on the front lines of the pandemic.
On April 3, the Department of Health (DOH) announced it would conduct “mass testing” for high-risk suspected COVID-19 carriers starting April 14.
The DOH has set protocols for coronavirus testing and classified patients with possible coronavirus infection in two: PUMs required to do a 14-day self-quarantine and PUIs who require hospitalization.
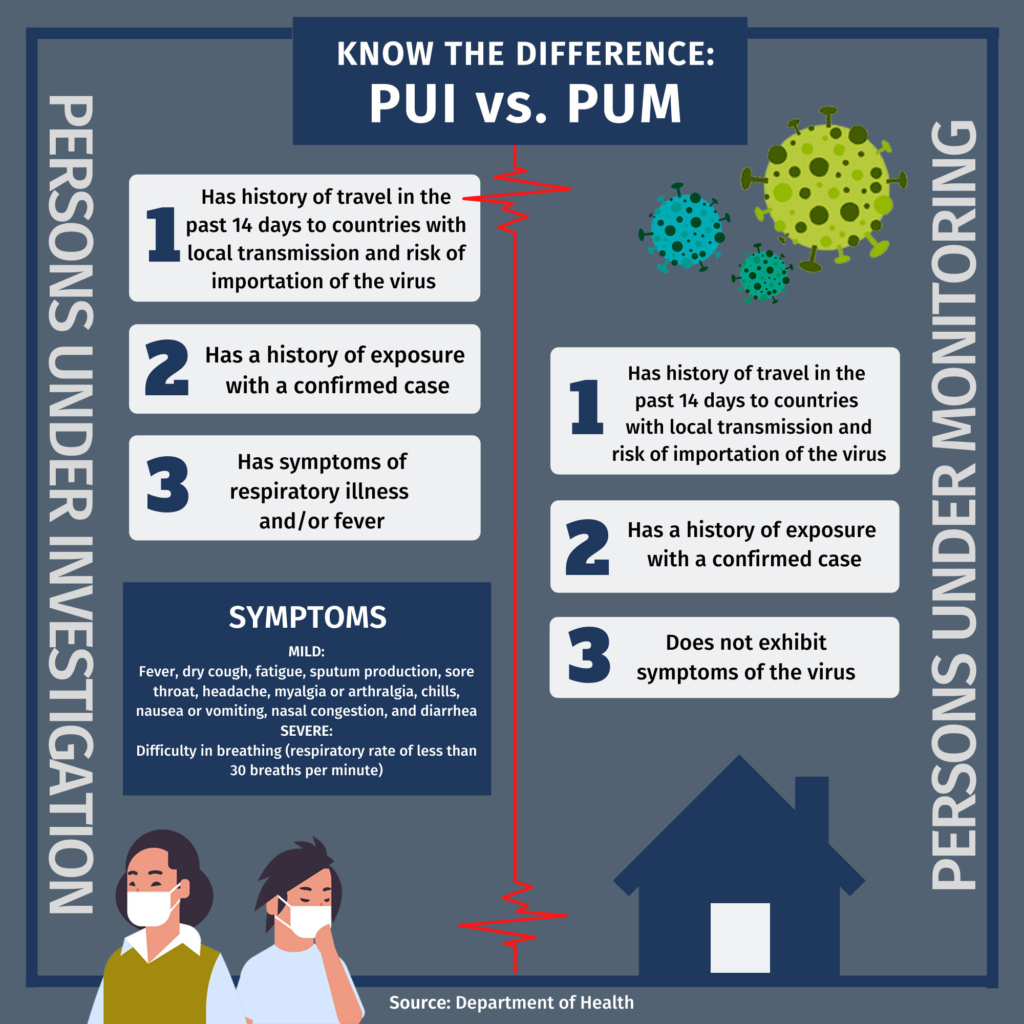
PUIs with severe manifestations of symptoms, along with the vulnerable such as the elderly, pregnant women and immunocompromised persons, will be prioritized for the said testing. DOH recently amended its protocols and included health workers with either mild or critical symptoms as subject to testing.
Next on the priority list are individuals showing mild symptoms, with irrelevant history of exposure or those of the vulnerable sector. They are followed by those who are asymptomatic or those without symptoms but have relevant travel history.
PH-made test kits
Last March, scientists from the Philippine Genome Center and the UP Manila National Institutes of Health developed the first locally made test kits, the GenAmplify coronavirus detection kit.
According to the Department of Science and Technology (DOST), the said test kit “aims to detect the 2019 novel coronavirus (SARS-CoV-2) with high specificity and efficiency through a one-step multiplex real-time polymerase chain reaction (PCR) platform.”
The homegrown kits look to accommodate 120,000 tests — 26,000 of which were funded by DOST, while the remaining 94,000 are sold commercially by Manila HealthTek for P1,320 per kit. This is about six times cheaper than its foreign counterparts which cost P8,500 each.
Additionally, the GenAmplify test kits are capable of quick detection of the virus in samples taken from patients. The results can be ready in two hours.
As of April 8, the UP-NIH-developed test kit is among the 23 PCR-based COVID-19 test kits approved by DOH for commercial use.
Testing is not an easy task
Persons qualified for testing undergo either a reverse transcription polymerase chain reaction (RT-PCR) test or a rapid antibody-based test.
An RT-PCR test is the most accurate test for COVID-19 and the virus SARS-CoV-2 that causes it, according to DOH.
The test starts with a “nasopharyngeal” swab, or a swab from the nose and throat. This swab collects mucus, saliva, bits of cells and, if present, viral RNA. Once the samples have been collected, they will be sent to a laboratory where scientists will apply certain chemicals to isolate the RNA.
RNA, the genetic material used by SARS-CoV-2, will then be “reverse transcribed” into DNA. The polymerase chain reaction will make many copies of a target DNA if present in a sample. According to Dr. Edsel Maurice Salvaña, an infectious diseases specialist, “it [RT-PCR test] is so sensitive that it can detect one virus in a sample.”
Salvaña said RT-PCR tests are “technically difficult to do” and a single PCR machine costs millions of pesos. But unlike GenAmplify, other RT-PCR tests take about three to six hours to run these tests and results can be expected in a day or two.
An alternative testing method for coronavirus is the rapid antibody-based method or immunologic method. Unlike RT-PCR tests, this testing method uses blood samples from a needlestick or a syringe extraction and yields results faster compared to genetic tests.
However, the immunologic method can only determine whether antibodies — proteins produced by the immune system to fight viruses — are present after the said symptoms are shown. It cannot detect the virus itself.
Two antibodies are detected through this test: IgM and IgG. IgM is produced earlier when someone is infected by the disease and can be detected in five to ten days from infection. Meanwhile, IgM is produced later and can take about 21 days before detection.
This kind of testing method usually yields inaccurate results as antibodies don’t develop immediately.
Food and Drug Association (FDA) Director General Eric Domingo said, “The body takes time to develop antibodies and this might give a negative result [known as false negative] for patients who have been infected but have not yet developed antibodies.”
“A positive result due to cross reaction with other bacteria or viruses is also possible, which is why a confirmatory PCR-based test is still required.”
Domingo said rapid antibody-based tests must be administered carefully and cautiously. “[Rapid test kits are] strictly for medical professional use only and not intended for personal use.”
“The administration of the test and interpretation of results should be done by a trained health professional,” he added.
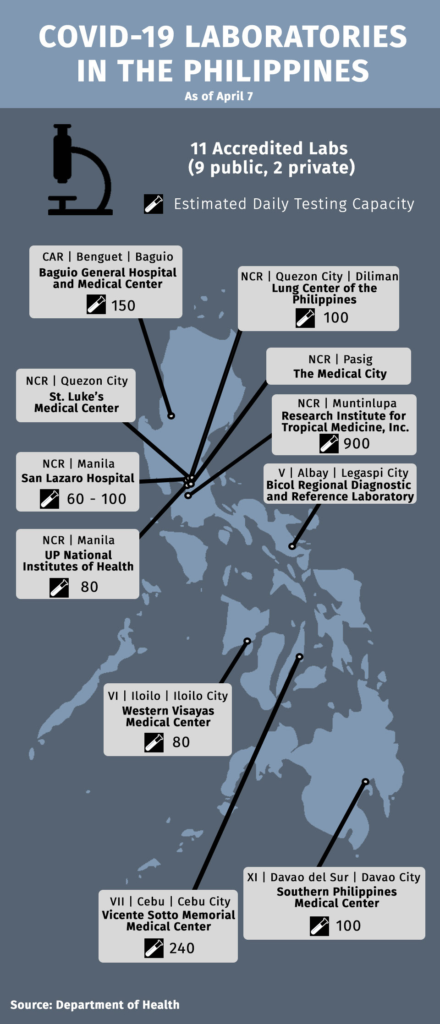
As of April 7, DOH has accredited eleven testing centers for COVID-19 testing: eight in Luzon, two in Visayas and one in Mindanao. Other testing centers have yet to be accredited.
As cases of COVID-19 continue to grow both in the country and globally, so too does the public’s collective fear and uncertainty.
But the arrival of more than a hundred thousand test kits, the accreditation of several COVID-19 testing centers, and the development of local kits in the country may give the country a chance to finally pursue “mass testing.”
Chelsea Cruz contributed research for this story.
This article first appeared in KRISIS (AY 2019-2020) – COVID-19 Pandemic Issue on April 12, 2020.





